THE 2017 18TH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS CONTENT IS NOW AVAILABLE IN VARIOUS POST CONFERENCE FORMATS
PHARMA CONGRESS CONTENT AS FOLLOWS:


Complete conference: $195
$195
KEYNOTE SPEAKERS

Thomas W. Abrams, RPh, MBA
Director, Office of Prescription Drug Promotion, US Food and Drug Administration, Silver Spring, MD

Tom Hubbard, MPP
Vice President of Policy Research, NEHI (The Network for Excellence in Health Innovation); Former Executive Assistant for Economic Affairs, US Senator John Kerry; Former Deputy Director of Development, Massachusetts Governor Michael Dukakis; Cambridge, MA

Mary E. Riordan, Esq.
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Washington, DC

James Sheehan, JD
Chief, Charities Bureau, Attorney General of New York; Former Chief Integrity Officer, New York City Human Resources Administration; Former, NY Medicaid Inspector General; Former AUSA, US Attorney’s Office, Eastern District of Pennsylvania; New York, NY

Jim Stansel, JD
Executive Vice President, General Counsel and Corporate Secretary, PhRMA; Former Acting General Counsel, US Department of Health and Human Services; Washington, DC
KEYNOTE DOJ CIVIL AND CRIMINAL DIVISIONS ROUNDTABLE

Colin M. Huntley, JD
Assistant Director, Civil Division, Fraud Section, U.S. Department of Justice, Washington DC

Pablo Quiñones, JD
Chief, Strategy, Policy and Training Unit, Fraud Section, Criminal Division, US Department of Justice; Former Assistant United States Attorney in the Criminal Division, US Attorney’s Office for the Southern District of New York; Washington, DC

Gejaa T. Gobena, JD
Partner, Hogan Lovells; Former Deputy Chief, Fraud Section, Criminal Division; Former Trial Attorney, Civil Division, Fraud Section, US Department of Justice; Washington, DC (Moderator)
KEYNOTE DOJ, SEC AND FBI FCPA ENFORCEMENT ROUNDTABLE

Charles Cain, Esq.
Deputy Chief, FCPA Unit, US Securities and Exchange Commission, Washington, DC

Tarek Helou, Esq.
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC

Darryl Wegner, JD
Unit Chief, International Corruption Unit, FBI, Washington, DC

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings; Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc.; New York, NY (Moderator)
KEYNOTE AUSA ROUNDTABLE

Jacob T. Elberg, JD
Chief, Health Care and Government Fraud Unit, United States Attorney’s Office, District of New Jersey, US Department of Justice, Newark, NJ

Jason Mehta, JD
Assistant US Attorney, United States Attorney’s Office Middle District of Florida, US Department of Justice, Jacksonville, FL

Gregg Shapiro, JD
Assistant US Attorney, US Attorney’s Office, District of Massachusetts, US Department of Justice, Boston, MA

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice; Washington, DC (Moderator)
KEYNOTE QUI TAM ROUNDTABLE

Amy L. Easton, JD
Attorney at Law, Phillips & Cohen, LLP, Former Senior Trial Counsel, Department of Justice, Washington, DC

Neil V. Getnick, JD
Managing Partner, Getnick & Getnick LLP; Former Assistant District Attorney, Manhattan District Attorney’s Office; New York, NY

Robert Patten, JD
President and Chief Executive Officer, Taxpayers Against Fraud and TAF Education Fund; Former Assistant Attorney General and Managing Attorney, Medicaid Fraud Division, Office of the Attorney General, Commonwealth of Massachusetts; Washington, DC

Virginia “Ginny” A. Gibson, Esq.
Partner, Hogan Lovells LLP; Former First Assistant U.S. Attorney, Eastern District of Pennsylvania, US Department of Justice; Philadelphia, PA (Co-Moderator)

Kathleen Meriwether, JD
Principal, Fraud Investigation & Dispute Services, Ernst & Young LLP; Former Assistant United States Attorney, Eastern District of Pennsylvania, US Department of Justice; Philadelphia, PA (Co-Moderator)
KEYNOTE DEMONSTRATING THE VALUE OF THE COMPLIANCE ORGANIZATION

Kathleen Boozang, JD LLM
Dean and Professor of Law, Seton Hall Law School, Newark, NJ

Michael Dusseau
Vice President, Compliance Operations, Allergan plc, New York, NY

Michael Shaw, JD
Vice President and Compliance Officer, US Pharmaceuticals, GlaxoSmithKline; Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services; Former Member, PCF Executive Committee; Philadelphia, PA

Erinn Hutchinson
Partner, Advisory Services, PwC, Philadelphia, PA (Moderator)
KEYNOTE CCO ROUNDTABLE

Jill Dailey, MBA, JD
Vice President and Chief Compliance Officer, Incyte; Former Assistant General Counsel and Asia Pacific Compliance Lead, Pfizer; Former Head of US Ethics and Compliance, Novartis; New York, NY
Sujata T. Dayal, JD
Vice President, Health Care Compliance & Privacy, Pharmaceuticals Group, Johnson & Johnson, Titusville, NJ

Margaret K. Feltz, MA, JD
Vice President, Chief Compliance Officer, Ethics and Compliance, Purdue Pharma LP; Former Member, PCF Executive Committee; Stamford, CT

Arjun Rajaratnam, JD, MS
Chief Compliance Officer, Smith & Nephew; Former Compliance Officer, US Pharmaceuticals, GlaxoSmithKline; Former Member, PCF Executive Committee; Raleigh, NC

Paul Silver
Principal, Regulatory & Compliance Life Sciences Leader, Deloitte Advisory, Deloitte, Atlanta, GA
FEATURING PRECONFERENCE SESSIONS
- Advanced Medical Affairs Compliance Issues
- The Basics of Managed Markets
- Medical Device Best Practice Compliance Updates
- Government Programs for the Compliance Officer
PLENARY SESSIONS
- OIG Compliance Update
- FDA Compliance Update
- DOJ Evaluation of Corporate Compliance Programs
- DOJ, SEC and FBI FCPA Enforcement Update
- AUSA Roundtable
- Chief Compliance Officer Roundtable
- Qui Tam Roundtable
- Demonstrating the Value of the Compliance Organization
- Role of Behavioral Economics in Ethics and Compliance
- PhRMA Priorities and Policy Initiatives
- Overview of the Policy and Politics of Pharma Pricing
- Rewarding Results: Value-Based Contracting for Biopharmaceuticals
AN INDUSTRY-ONLY COMPLIANCE BEST PRACTICES THINK TANK
AN INVITATION-ONLY CHIEF COMPLIANCE OFFICER ROUNDTABLE
AND MINI SUMMITS
- Using Behavioral Economics to Improve Compliance Messaging Stickiness
- HCP Engagement: The Road to Proactive Risk Management
- Best Practices for Patient Assistance and Reimbursement Support
- Managed Market Considerations for Hub and Specialty Pharmacy Arrangements
- Compliance and Ethics Consideration in R&D
- Pricing Considerations (Mylan Epipen, Marathon Pharmaceuticals, et al)
- International Organization for Standardization (ISO) 37001
- FDA Regulation and Cutting Edge Technology
- Advancing Compliance: Pulling Critical Levers to Improve Effectiveness
- The Evolution of Compliance Programs after Wells Fargo
- Innovation and Compliance, They are not Mutually Exclusive
- Third Party Risk Management/Due Diligence Update
- Compliance in the Transactional Context
- Managing an Internal Investigation under CIA, DPA and Data Analytics Failure
- Recent FDA Guidance: Manufacturer Communications and Off-Label Memo
- Small and Mid-Sized Pharma and Device Companies Compliance Considerations
- Advanced Issues in Global Compliance
- Effective and Balanced Monitoring Program based on Risk
- Advanced Compliance Issues When Contracting with Third Parties
- The Next Generation of Agg Spend Solutions
- Cyber Security for Pharma and Medical Device Companies
- Using Data Analytics to Tell the Compliance Effectiveness Story
- Optimizing Data Collection for Risk Assessments, Monitoring, and Auditing
- Balancing Risks in Patient and Product Support
FEATURED FACULTY

Sergio Alegre, JD
Vice President, Global Compliance, Osmotica Pharmaceutical Corp.; Former Executive Director Compliance, Pacira Pharmaceuticals, Inc.; New York, NY

Anthony L. Alvizu
Managing Director, Global Risk & Investigations Practice, FTI Consulting, Chicago, IL

Yogesh Bahl, CPA, MBA
Managing Director, Alix Partners, New York, NY

Virginia Beakes-Read, JD
Executive Director/Special Counsel, Regulatory Strategy and Law, Eisai Inc., Washington DC

Ann Beasley, JD
Director, Navigant Consulting; Former Senior Vice President, Chief Compliance Officer, Biogen; Boston, MA

Thomas W. Beimers, Esq.
Partner, Hogan Lovells; Former Senior Counsel for Administrative and Civil Remedies, Office of the Inspector General, Department of Health and Human Services; Minneapolis, MN

Andy Bender, MS, MBA
General Manager, QuintilesIMS, New York, NY

Raymond A. Bonner, JD
Partner and Chair, Food, Drug and Medical Device Compliance and Enforcement Practice, Sidley Austin LLP; Former Assistant US Attorney, US Attorney’s Office, District of Maryland; Washington, DC

Kenneth Borgerding, JD
Vice President, Chief Compliance Officer, Lundbeck; Deerfield, IL

Anthony Brennan, MBA
Senior Manager, Fraud Investigation and Dispute Services, Ernest & Young; Former Senior Director, Governance, Metrics and Reporting, Health Care Compliance, Johnson & Johnson; Iselin, NJ

Thomas G.A. Brown, JD
Managing Director, Global Practice Leader – Cyber Security & Investigations, Berkeley Research Group; Former AUSA, U.S. Attorney’s Office for the Southern District of New York; New York, NY

Katherine Buckley, MBA
Principal, Pharmaceutical and Life Sciences Advisory Practice, PwC, Philadelphia, PA

Terra Buckley
Senior Director, US HCC, I&I Franchise, Global Compliance, Celgene Corporation, Summit, NJ

Christie Camelio
Vice President and US Healthcare Compliance Officer, Celgene Corporation, Summit, NJ

Jennifer Chillas, JD
Senior Corporate Counsel, Bristol-Myers Squibb, Princeton, NJ

Robert F. Church, JD
Partner, Hogan Lovells US LLP; Former Associate General Counsel, Amgen; Former Associate Chief Counsel, FDA; Los Angeles, CA

Andrew T. Clark, MBA
Partner/Principal, EY, Washington DC

Jim Clement, MHA
Executive Director, Cost of Care and Supply Chain Strategy, Aetna; Former National Account Manager, PBMs and Specialty Pharmacy, Genentech, Inc.; Former Director, Pharmaceutical Contracting, Express Scripts, Inc.; Hartford, CT

Kellie B. Combs, JD
Partner, Ropes & Gray, Washington, DC

Joseph Coniker
Principal, Enterprise Performance Management (EPM) Analytics, Grant Thornton LLP, Raleigh, NC

Brian Conner
Vice President, Head of Corporate Compliance, Strongbridge Biopharma plc, Trevose, PA

Tom Costa, JD
Pharmaceutical Consultant, Aventis Board of Directors; Former Vice President, US Compliance & Ethics, Bristol-Myers Squibb; Washington, DC

Clarissa Crain
Senior Manager, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Philadelphia, PA

David Cromley, JD
Associate Vice President, Merck, Philadelphia, PA

Jodi G. Daniel, MPH, JD
Partner, Crowell & Morings, Washington DC

Meenakshi Datta, JD
Partner, Sidley Austin LLP, Chicago, IL

BJ D’Avella
Senior Manager, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Atlanta, GA

Gary Del Vecchio
Health Care Compliance Officer Cardiovascular & Metabolism and Puerto Rico, The Janssen Pharmaceutical Companies of Johnson & Johnson; Former, Executive Director, US Pharmaceutical Compliance & Ethics, Bristol-Myers Squibb; Titusville, NJ

Mark A. DeWyngaert, MBA, PhD
Managing Director, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, New York, NY

Sarah diFrancesca, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP, New York, NY

Stefanie A. Doebler, JD, MPH, MA
Of Counsel, Covington & Burling LLP, Washington, DC

Michelle Drozd, ScM
Deputy Vice President, Policy and Research, Pharmaceutical Research & Manufacturers of America (PhRMA), Washington, DC

Corey Dunbar
Senior Manager, Fraud Investigations & Dispute Services, EY, Iselin, NJ

Gildas Durand
Partner/Principal, Fraud Investigation and Internal Audit, EY, Miami, FL

Liisa Eisenlohr, PhD, MBA
Associate Director, Navigant; Former Senior Director, Global Medical Information, Clovis Oncology; Former Clinical Scientist, Genentech; San Francisco, CA

Megan Falkowski
Director, Government Pricing & Government Contracting Policy, Pfizer Inc., New York, NY

Jill Fallows-Macaluso, JD
Chief Compliance Officer and Vice President, Novo-Nordisk, Princeton, NJ

David J. Farber, JD
Partner, Healthcare Lobbyist and Litigator, King & Spalding LLP, Washington, DC

Alison Fethke, JD
Counsel, Ropes & Gray LLP; Former Division Counsel, Legal, Regulatory and Compliance, AbbVie; Chicago, IL

Chris Fletchall, MBA
Senior Director, Ethics and Compliance, Global Operations, Eli Lilly and Company, Indianapolis, IN

Sarah A. Franklin, JD
Partner, Covington & Burling, LLP, Washington DC

Nereyda Garcia, JD
V.P., Global Heath, Ethics and Compliance, Alnylam Pharmaceuticals, Alnylam Pharmaceuticals; Former Sr. Director, Compliance, Biogen Idec; Boston, MA

Thomas M. Glavin, JD
Chief Compliance Officer, Olympus Corporation of the Americas; Former Vice President, US/Americas Compliance Officer, Shire; Center Valley, PA

Jonathan Glazier, JD, MBA
Senior Legal Counsel, US Compliance Lead, Philips Electronics North America; Former Senior Director of Corporate Compliance and Privacy Officer, Fresenius Medical Care North America; Andover, MA

Wendy C. Goldstein, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP, New York, NY

Amy Gregory, CCEP
Compliance Director, AstraZeneca, Gaithersburg, MD

David Hartenbaum, MD, MBA
Executive Director, Account Management, Merck, Philadelphia, PA

Lindsay Havern, JD
Vice President and Assistant General Counsel, Pfizer, New York, NY

Justin Herring, JD
Assistant United States Attorney, United States Attorney’s Office, District of New Jersey, US Department of Justice, Newark, NJ

Kristin M. Hicks, JD
Partner, Arnold & Porter Kaye Scholer LLP, Washington, DC

Laura G. Hoey, JD
Partner, Government Enforcement Group, Ropes & Gray LLP; Former Assistant US Attorney, US Attorney’s Office, Eastern District of Arkansas, US Department of Justice; Chicago, IL

Dorothy Hoffman, MPP
Director, Healthcare Transformation and Policy Partnerships, Eli Lilly and Company, Indianapolis, IN

Casey J. Horton, CFE
Director, Healthcare and Life Sciences Disputes, Regulatory, Compliance and Investigations, Navigant Consulting, Chicago, IL

William Hrubes, MHA
Vice President, Chief Compliance Officer, ACell, Inc., Columbia, MD

William J. Hughes, Jr., JD, LLM
Principal, Porzio, Bromberg & Newman, PC; Former Assistant US Attorney and Trial Attorney, US Department of Justice; Morristown, NJ

Darren R. Jones, CIA
Managing Partner, Polaris Management Partners, New York, NY

Catherine Healy Kanzler, JD
Executive Director, Global Compliance Operations, Olympus, Center Valley, PA

Gary Keilty
Managing Director, FTI Consulting, Tampa, FL

Anup Kharode, MS, MBA
Partner, Pharmaceutical & Life Sciences R&D Advisory Services, PwC, Philadelphia, PA

John Knighton, JD
Vice President, Head of Global Compliance, Orexigen Therapeutic; Former Vice President, CCO, MicroPort Medical (Group) Co., Ltd.; San Diego, CA

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC

Daniel A. Kracov, JD
Partner and Head, FDA and Healthcare Practice, Arnold & Porter Kaye Scholer LLP, Washington, DC

Holly Kramen, JD
Former Vice President, Global Compliance Officer, Circassia, Washington, DC

Monica Kwarcinski, PharmD
Head of External Medical Affairs, Purdue Pharma LP, Stamford, CT

Mark Lange, JD
Assistant General Counsel, Eli Lilly and Company, Indianapolis, IN

Jonathan Levy, JD
Corporate Compliance Lead, Spark Therapeutics; Former Associate Director of Compliance and Ethics, US Pharmaceuticals, Bristol-Myers Squibb; Philadelphia, PA

Maureen Lloyd
Director Pharmaceutical and Life Sciences, PwC; Former Executive Director, Medical – External Medical Communications, Pfizer Inc.; New York, NY

Pamela Lonzer, MJ
US Medical Advisor, Shire; Former Associate Director, Global Medical Affairs Compliance, Baxalta; Former Assistant Director, R&D-Global Compliance, Astellas Pharma; Chicago, IL

Michael K. Loucks, JD
Partner, Skadden Arps LLP; Former Acting United States Attorney, District of Massachusetts, United States Department of Justice; Washington, DC

Chia-Feng Lu, JD
Associate, Baker McKenzie, Washington, DC

Seth H. Lundy, JD
Partner, King & Spalding, Washington, DC

Brian Miller, MS, PhD Candidate
Senior Director of Compliance Training and Documentation, Otsuka Pharmaceutical Companies, Senior Manager of Learning Technologies, AstraZeneca; Former Associate Director Learning Design & Technology, Merck; Philadelphia, PA

Teresa Muckel
Associate Director, Government Price Reporting, Genentech, USA, South San Francisco, CA

Michelle Murphy
Director Compliance, Regeneron Pharmaceuticals, Inc., Tarrytown, NY

Meghan Naas, MA
Senior Finance Manager, Compliance TESARO, Inc., Waltham, MA

Katherine (Katie) Norris, MPA
Director, Corporate Compliance & Risk Management, BRG; Former Director, Corporate Compliance, Spectranetics; Washington, DC

Michael O’Connor, MS
Independent Consultant; Former Executive Director, Global Head, Boehringer Ingelheim; Former Senior Director, Global Head Compliance and Ethics Operations, Alexion Pharmaceuticals, Inc., New York, NY

William P. Olsen
Principal, Practice Leader, Corporate Compliance, Forensic Advisory Services, Grant Thornton LLP, Arlington, VA

John Patrick Oroho, JD
Executive Vice President, and Chief Strategy Officer, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ

Becky Osowski, MJ
Senior Manager, Fraud Investigation and Dispute Services Practice; Former US Healthcare Compliance Officer and Deputy Compliance Officer, Zimmer; Chicago, IL

Mona M. Patel, JD
Partner, Covington & Burling LLP, Washington DC

Amy Pawloski
Global Lead, Compliance Risk Mitigation and Monitoring Strategy, Bristol-Myers Squibb, Philadelphia, PA

Paul J. Peterson, CPA, CIA, CFE, CFF
Senior Manager, Forensic Advisory Services, Grant Thornton, Arlington, VA

Kathryn Pryze, CCEP
Compliance Director, AstraZeneca, Wilmington, DE

Matt Queler, JD
Principal, Deloitte Risk & Financial Advisory; Former Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice; Former Assistant U.S. Attorney, US Attorney’s Office, District of New Jersey, Washington, DC

John Seungjoo Rah, JD
Partner, DLA Piper, Washington, DC

Louis Ramos, JD
Partner, DLA Piper; Former Assistant General Counsel, Compliance Division, Pfizer; Former Assistant U.S. Attorney, US Attorney’s Office, District of Columbia, US Department of Justice; Washington, DC

Kelly N. “Nikki” Reeves, MPA, JD
Partner, King & Spalding LLP, Washington, DC

Megan Reiss, LLM, PhD
Senior National Security Fellow, R Street Institute, Washington DC

Beth Richardson
Director, Compliance, Horizon Pharma, Inc.; Former Compliance Director, CVS Health; Lake Forest, IL

Ron Sandhu
Senior Manager, Deloitte Advisory, Deloitte & Touche LLP, New York, NY

Jennifer Sanfilippo, JD
Vice President, Commercial Integrity Counsel, The Medicines Company, Parsippany, NJ

Sue Seferian, JD
Health Care Compliance Officer, Johnson & Johnson, Titusville, NJ

John D. Shakow, JD
Partner, King & Spalding LLP, Washington, DC

Brian Sharkey, JD
Porzio, Bromberg & Newman, PC, Morristown, NJ

Greg Sherman, JD
Commercial Counsel, Gilead Sciences, Foster City, CA

Michelle M. Shwery, MSc, MBA
Senior Advisor, Ethics and Compliance, Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN

Laura Skinner
Senior Manager, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Austin, TX

Jon Smollen, MA, JD
Practice Professor of Law and Director, Center for Compliance and Ethics, Temple Law School; Former Executive Vice President and Chief Compliance Officer, Endo International; Philadelphia, PA

Mariann Snyder, CCEP
Global Compliance Communications Lead, AstraZeneca, Wilmington, DE

Daniel Spicehandler, JD
Director, Risk and Accountability, Novo Nordisk, New York, NY

Oliver Steck
Principal, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, New York, NY

Tracy Strong, JD
Vice President, International Compliance Officer, Laboratory Corporation of America, Burlington, NC

Cortnaye Swan
Sr. Manager, Deloitte & Touche LLP, Kansas City, MO

Jack Tanselle, MBA
Managing Director, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Indianapolis, IN

Ann-Marie Tejcek, MA
Senior Director Ethics & Compliance, USA/Canada, Eli Lilly and Company, Indianapolis, IN

Bryan Timer
Director, Data Analytics & Transparency, Merck, Allentown, PA

Andrew R. Van Haute, JD
Associate, Sidley Austin LLP; Former Associate General Counsel, AdvaMed; Washington, DC

Brian Van Hoy
Vice President, Compliance, G & M Health LLC; Former Director, Ethics and Compliance, Eli Lilly; Bridgewater, NJ

Julie Ritchie Wagner, JD
Assistant General Counsel, PhRMA; Former Senior Counsel, Office of Counsel to the Inspector General, US Department of Health and Human Services; Washington, DC

Lisa Walkush
Principal, Advisory Services; National Life Sciences Sector Leader, Grant Thornton LLP, Philadelphia, PA

Matt Wetzel, JD
Vice President and Assistant General Counsel, Advanced Medical Technology Association (AdvaMed); Former Senior Counsel, Global Compliance and Ethics, Boston Scientific; Washington, DC

Donna White, CCEP
Vice President, Contracts & Compliance, Chiesi USA, Inc.; Former Sr. Director, Contracts and Compliance, Cornerstone Therapeutics; Cary, NC

Seth B. Whitelaw, JD, LLM, SJD
President and Chief Executive Officer, Whitelaw Compliance Group, LLC; Editor, Life Science Compliance Update; Senior Fellow & Adjunct Professor, Life Sciences Compliance at Mitchell Hamline School of Law, West Chester, PA

Jane H. Yoon, JD
Of Counsel, Litigation Department, Paul Hastings; Former Assistant United States Attorney, Health Care and Government Fraud Unit, US Attorney’s Office for the District of New Jersey, US Department of Justice; New York, NY
2017-2018 GLOBAL PHARMA COMPLIANCE CONGRESSES
EIGHTEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum (PCF)
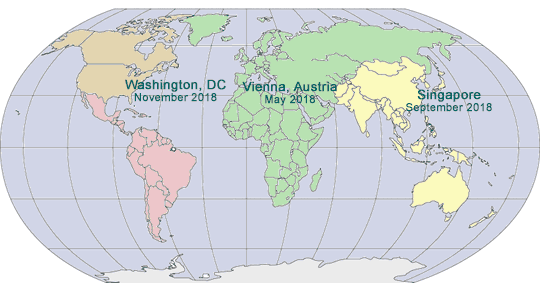
November 6 – 8, 2017
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
TWELFTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by Pharmaceutical Compliance Forum (PCF)
Media Partners: Life Sciences Compliance Update
May 14 – 16, 2018
Hotel Savoyen
Vienna, Austria
www.InternationalPharmaCongress.com
EIGHTH ASIA PACIFIC PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
Media Partners: Life Sciences Compliance Update
September 2018
Singapore
www.AsianPharmaCongress.com
INDIAN PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
International Society of Health-
care Compliance Professionals (ETHICS)
Cosponsored by International Society of Healthcare Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
Fall 2018
Mumbai, India
Coming Soon


